Amazing Manchester research leads to life-saving treatment for babies with rare disease
- Written by I Love MCR
- Last updated 1 year ago
- Charity, Civic, People
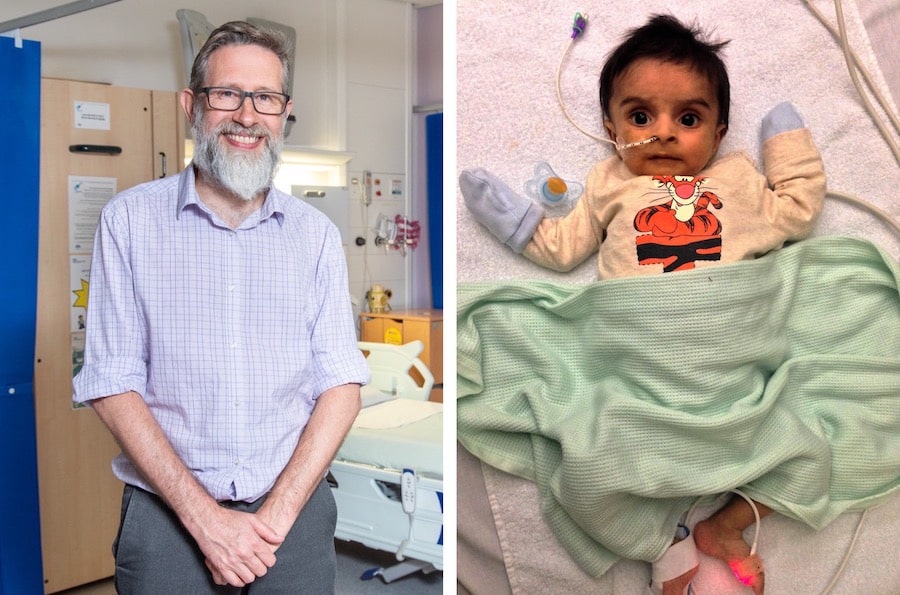
Babies and toddlers with a rare and fatal genetic condition can now receive life-saving treatment on the NHS for the first time thanks to clinical research in Manchester.
This follows clinical research studies first carried out at the National Institute for Health and Care Research (NIHR) Manchester Clinical Research Facility (CRF) at Royal Manchester Children’s Hospital (RMCH) in collaboration with the Manchester Centre for Genomic Medicine at Saint Mary’s Hospital, both part of Manchester University NHS Foundation Trust (MFT).
An enzyme replacement therapy, sebelipase alfa (Kanuma), is the first treatment available on the NHS for lysosomal acid lipase deficiency, also known as Wolman disease, a condition which presents in babies and children under two years old.

Sebelipase alfa was recommended today (Monday 27 November) for use on the NHS by the National Institute for Health and Care Excellence (NICE) for the treatment of Wolman disease.
The NIHR Manchester CRF at RMCH delivered world-first clinical trials for the treatment, which began in May 2011. The facility undertook phase 1 and phase 2 studies which involved several different specialist teams working collaboratively, over a number of years.
MFT has been a trailblazer in this field over the last decade, working closely as a multi-disciplinary team to provide the best treatment, care and innovative research for babies born with Wolman disease. To date, the team in Manchester have delivered life-saving treatment to the most children globally.

This includes Hashir Nawaz, eight-years-old, from Sheffield, South Yorkshire, who was diagnosed with Wolman disease aged three months. Hashir started treatment with sebelipase alfa in January 2016, first as part of a clinical trial at the NIHR Manchester CRF at RMCH, and then via a compassionate access scheme (access to the medicine outside of a clinical trial). Hashir receives the treatment once every two weeks.

Hashir’s father, Jabran, said: “Without sebelipase alfa, Hashir would not be alive today. Thanks to the treatment, Hashir turned eight last month and is able to live a normal life – going to school full-time, meeting friends on the weekend, and enjoying holidays abroad, including Disneyland. This medicine has made a huge difference to our lives, and we are incredibly grateful to the clinicians and the hospital for Hashir’s treatment.”
Wolman disease is a rapidly progressing and life-threatening rare, genetic condition that causes multi-organ damage. It causes a build-up of fat in cells in the liver, heart, blood vessels, and digestive system. Without treatment, infants with Wolman disease normally do not live to see their first birthday.
Professor Simon Jones (main picture), Consultant in Paediatric Inherited Metabolic Disease at the Manchester Centre for Genomic Medicine at Saint Mary’s Hospital and Clinical Director of NIHR Manchester CRF at RMCH, said: “Today marks a major milestone in treatment for infants born with Wolman disease and signifies a substantial step forward in our dedication to practical advancements in rare disease medicine and improved patient outcomes, through research.
“I am thrilled to see that this lifesaving drug will now be available on the NHS as a specialist service for the benefit of more children and families with this rare genetic condition. More than a decade on since our world-first clinical trials, I am incredibly proud of what our research and clinical teams here in Manchester have delivered collaboratively, which has contributed to this successful outcome.”
There were previously no treatment options for Wolman disease on the NHS, and standard care was palliative and limited to managing symptoms, but thanks to a commercial deal, NHS England will fast-track this life-saving treatment for patients by utilising the Innovative Medicines Fund (IMF).

Liz Worsley (above), Nurse Manager at the NIHR Manchester CRF at RMCH, gave the first dose of the treatment in the world-first phase 1 trial in 2011, when she was a senior clinical research nurse.
She said; “It is absolutely amazing to see the difference this treatment has made. Particularly when I see some of these children in the hospital coming to their appointments now thriving, attending school and achieving everything you would want for your own child, it is really rewarding.
“It’s so heart-warming when the families remember us, and it’s been a real privilege to be a part of this chapter in their lives and to share this journey with them.”
Wolman’s disease is rare and occurs in around 1 in 350,000 births. It is caused by mutations in a gene and is an inherited, recessive condition. It is estimated that around one or two babies born every year in England will have the disease.
Symptoms in babies include enlarged liver and spleen, poor weight gain, low muscle tone, jaundice, vomiting, diarrhoea, anaemia, and malabsorption (difficulty in the digestion or absorption of nutrients from food).
Sebelipase alfa is used as an enzyme replacement therapy, which works by replacing an enzyme missing in the body, alongside a restricted, low-fat diet. Treatment involves weekly intravenous infusions which can be given at home – some patients may also have a blood and marrow/stem cell transplant.

Fiona White (above) is the Lead Specialist Metabolic Dietitian at the Manchester Centre for Genomic Medicineand has played a vital role in ensuring that children receiving treatment for Wolman disease are on a specialised low-fat diet which is imperative for their growth and development.
Infants who have Wolman disease struggle to break down fats. Fats then build up in parts of the body such as the gut. This causes the gut to stop working as it should and as a result infants get severe vomiting and diarrhoea and are unable to gain weight as they should. Using a very low-fat diet is critical for these patients. Through this research, Fiona and her team have been able to learn and understand what nourishment children with this disease can tolerate and have been able to refine a specialised diet which contains almost zero fat. With this diet the children have grown normally.
She said: “It has been an amazing journey to get to this point and a real team effort, which I am incredibly proud of. Our knowledge in this area has expanded beyond expectation and it’s very rewarding to see how the children continue to thrive and are enjoying life just as they should.
“Before this treatment, children with Wolman disease died early and so our knowledge in this area was limited. We have had to adapt and innovate to build up our expertise in ensuring they are getting the right nutrients in their diet to grow and develop, providing an individualised plan for each child.
“To see this treatment available on the NHS is fantastic and provides real hope for families and infants diagnosed with this devastating disease. It’s very rewarding to see how our investment into this research has paid off and we have shared our knowledge with hospitals globally.”
Shoaib, eight, from Halifax, started treatment with sebelipase alfa when he was three days old. Having had an older brother sadly pass away from Wolman disease, Shoaib was diagnosed with the rare condition at birth. By this time, sebelipase alfa was now available as part of a clinical trial at the NIHR Manchester CRF at RMCH, and Shoaib was able to benefit. After being treated with the drug for over two years, Shoaib then received a haematopoietic stem cell transplant, meaning he no longer requires treatment with sebelipase alfa.
Shoaib’s mother, Nadia, said: “When Shoaib was diagnosed with Wolman disease we were incredibly worried as we’d already lost another child to the illness. However, the treatment gave us real hope for the future, enabling Shoaib to live a normal life where he can go to school every day. The treatment was fantastic, and I’m thrilled that more patients will now be able to access it through the NHS.”
Professor Simon Jones continued: “Both Shoaib and Hashir received sebelipase alfa through clinical trials at the NIHR Manchester Clinical Research Facility at Royal Manchester Children’s Hospital, in collaboration with the Manchester Centre for Genomic Medicine at Saint Mary’s Hospital. It is extremely rewarding to see how life-changing this treatment has been for them and their families, and we are pleased to provide this specialist service to more infants, as one of three sites delivering the therapy in England.”
Up to £340 million was made available through the IMF, which launched last year, to purchase the most promising medicines and fast-track them to patients to give people the best chances of survival, or a healthier, longer life. Under the arrangements, sebelipase alfa will not enter managed access, but interim funding will be provided from the IMF to enable routine patient access up to five months earlier than would otherwise be the case.
The drug, which has been manufactured by Alexion, AstraZeneca’s Rare Disease group, has been recommended for use by NICE today in its final draft guidance. Through the IMF, treatment can be funded for eligible patients straight away.
Clinical trials have shown this treatment improves a patient’s life expectancy and quality of life.
Sebelipase alfa will be delivered by specialist services at MFT, Birmingham Women’s and Children’s Hospital and Great Ormond Street Hospital.
John Stewart, National Director for Specialised Commissioning at NHS England said:“It’s fantastic news that babies born with Wolman disease will be eligible to receive this life-saving treatment on the NHS from today. Where previously there were no treatments available for infants facing this debilitating and fatal disease, this new treatment could save families from facing indescribable grief and allow more children like Hashir and Shoaib to grow up, go to school and live normal lives.
“This is just the latest example of patients benefiting from innovative medicines secured through NHS commercial deals, following shortly after a new five-year medicines agreement was reached with the pharmaceutical industry that will enable rapid patient access to the treatments of tomorrow.”
- This article was last updated 1 year ago.
- It was first published on 27 November 2023 and is subject to be updated from time to time. Please refresh or return to see the latest version.
Did we miss something? Let us know: [email protected]
Want to be the first to receive all the latest news stories, what’s on and events from the heart of Manchester? Sign up here.
Manchester is a successful city, but many people suffer. I Love Manchester helps raise awareness and funds to help improve the lives and prospects of people across Greater Manchester – and we can’t do it without your help. So please support us with what you can so we can continue to spread the love. Thank you in advance!
An email you’ll love. Subscribe to our newsletter to get the latest news stories delivered direct to your inbox.
Got a story worth sharing?
What’s the story? We are all ears when it comes to positive news and inspiring stories. You can send story ideas to [email protected]
While we can’t guarantee to publish everything, we will always consider any enquiry or idea that promotes:
- Independent new openings
- Human interest
- Not-for-profit organisations
- Community Interest Companies (CiCs) and projects
- Charities and charitable initiatives
- Affordability and offers saving people over 20%
For anything else, don’t hesitate to get in touch with us about advertorials (from £350+VAT) and advertising opportunities: [email protected]

Hilton Manchester Deansgate’s superstar chefs shine on the world stage

Stalybridge to get multi-million pound makeover of public spaces

Review: The Engagement Party at Queen Elizabeth Hall ‘is full of immersive fun’

Piehard: where to get the best pies in and around Manchester

















